While the exact material ratios vary by manufacturer the common combinations are usually 60 nickel 20 manganese and 20 cobalt.
Lithium cobalt oxide battery chemical reaction.
Lithium nickel cobalt aluminum oxide linicoalo 2 nca.
Stanley whittingham rachid.
The chemical reaction is prompted because of the.
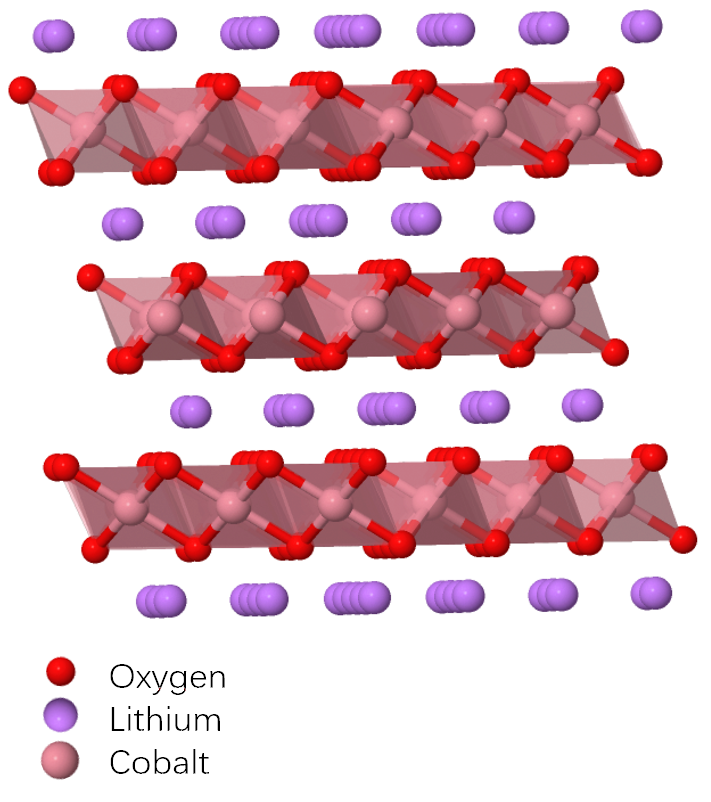
The cobalt and oxygen bond together to form layers of.
Lithium cobalt oxide is a dark blue or bluish gray crystalline solid and is commonly used in the positive electrodes of lithium ion batteries.
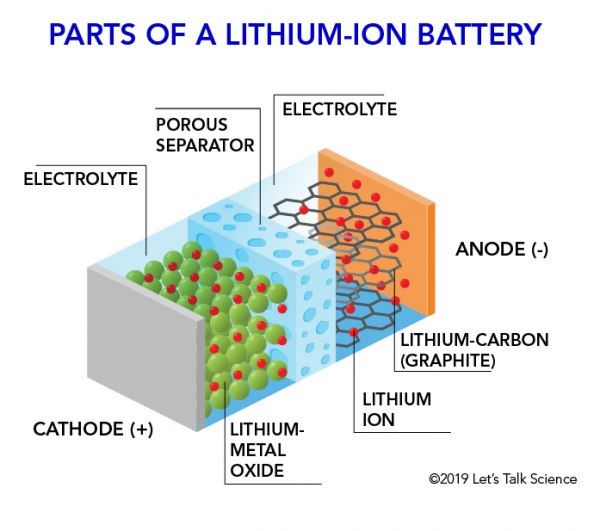
The positive electrode is typically made from a chemical compound called lithium cobalt oxide licoo 2 or in newer batteries from lithium iron phosphate lifepo 4.
So for lithium cobalt oxide batteries with liquid electrolyte i was able to find a good source describing the problem of thermal runaway.
In fact the lithium cobalt oxide battery was the first lithium ion battery to be developed from the pioneering work of r yazami and j goodenough and sold by sony in 1991.
Less flattering are safety and cost.
Recently strong demands for the quick renewal of the properties of electronic products ever.
A lithium ion battery or li ion battery abbreviated as lib is a type of rechargeable battery lithium ion batteries are commonly used for portable electronics and electric vehicles and are growing in popularity for military and aerospace applications.
By breaking through the energy density limits step by step the use of lithium cobalt oxide based li ion batteries lco based libs has led to the unprecedented success of consumer electronics over the past 27 years.
A prototype li ion battery was developed by akira yoshino in 1985 based on earlier research by john goodenough m.
The temperature of the cell rises due to the chemical reactions between the organic solvent and electrode materials leading to cell failure explosion.
Currently the most popular lithium ion technology is the lithium cobalt oxide lco battery which has a cathode composed of licoo 2 the main feature of the lco battery is the high energy density translating into a long run time for portable devices such as cell phones tablets laptops and cameras.
The most common lithium ion cells have an anode of carbon c and a cathode of lithium cobalt oxide licoo 2.
When charging the battery the breakdown of lithium cobalt oxide occurs li ions travel through electrolyte solution and bond to negatively charged anode this is because when charging electrons are sent to graphite causing a negative charge and attraction to li ions.
Lithium nickel cobalt aluminum oxide battery or nca has been around since 1999 for special applications.
The negative electrode is generally made from carbon graphite and the electrolyte varies from one type of battery to another but isn t too important in understanding the.